Metal halogen exchange
Home » Query » Metal halogen exchangeYour Metal halogen exchange images are ready. Metal halogen exchange are a topic that is being searched for and liked by netizens today. You can Get the Metal halogen exchange files here. Get all royalty-free photos and vectors.
If you’re looking for metal halogen exchange images information linked to the metal halogen exchange keyword, you have pay a visit to the right site. Our website always provides you with hints for seeing the maximum quality video and image content, please kindly search and find more informative video content and images that match your interests.
Metal Halogen Exchange. Transition-Metal-Catalyzed HalogenZinc Exchange Reactions. The reaction commonly involves the use of electropositive metals Li Na Mg and organochlorides bromides and iodides. Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi. However we have observed a.
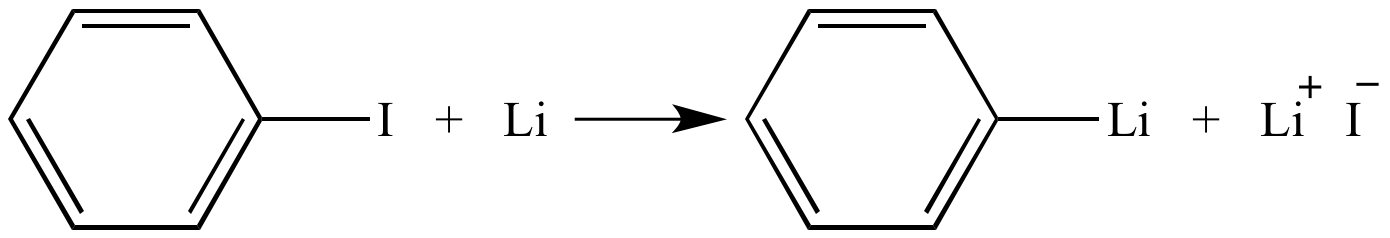
 The Metal Halogen Exchange Interaction Of Phenyllithium With Iodobenzene From www2.chem.wisc.edu
The Metal Halogen Exchange Interaction Of Phenyllithium With Iodobenzene From www2.chem.wisc.edu
When Mg is added to tert-butyl bromide the carbon-bromine bond is exchanged for a carbon-magnesium bond. Remarkably efficient processes have been developed for metal-mediated halogen exchange in aryl and vinyl halides. Particularly well-developed is the use of metalhalogen exchange for the preparation of organolithium compounds. H Br OEt H H Li OEt 11 eq n-BuLi H Et2O 80 C Lau K. In some instances the rate of lithium-halogen exchange can exceed the rate of. Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi.
R1X LiR2 LiR1 R2X R1Li R2X FourÐcentered transition state model LiR2 R1X.
Lithium-halogen exchange is extremely fast. Utilising the racemic phenoxide ligand 5566-tetramethyl-33-di-tert-butyl-11-biphenyl-22-diol rac-BIPHEN-H2 the dialkyl sodium magnesiates rac-BIPHENNa2MgBu2TMEDA23 and rac-BIPHENNa2MgBu2P. When the metalhalogen exchange temperature was set to 78 C. Perhaps the most logical approach is a typical cross-coupling. Magnesium-Halogen Exchange Chem 115 Jason Brubaker Review. In some instances the rate of lithium-halogen exchange can exceed the rate of.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
H Br OEt H H Li OEt 11 eq n-BuLi H Et2O 80 C Lau K. The yields of the newly formed Grignard and the final product are substantially improved in comparison to the identical reaction without a ligand additive. Aryl and Heteroaryllithium Compounds by Metal - Halogen Exchange. Of n-butyl-lithium in ether or THF under various reaction conditions followed by addition of dimethyl sulphate. Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi.
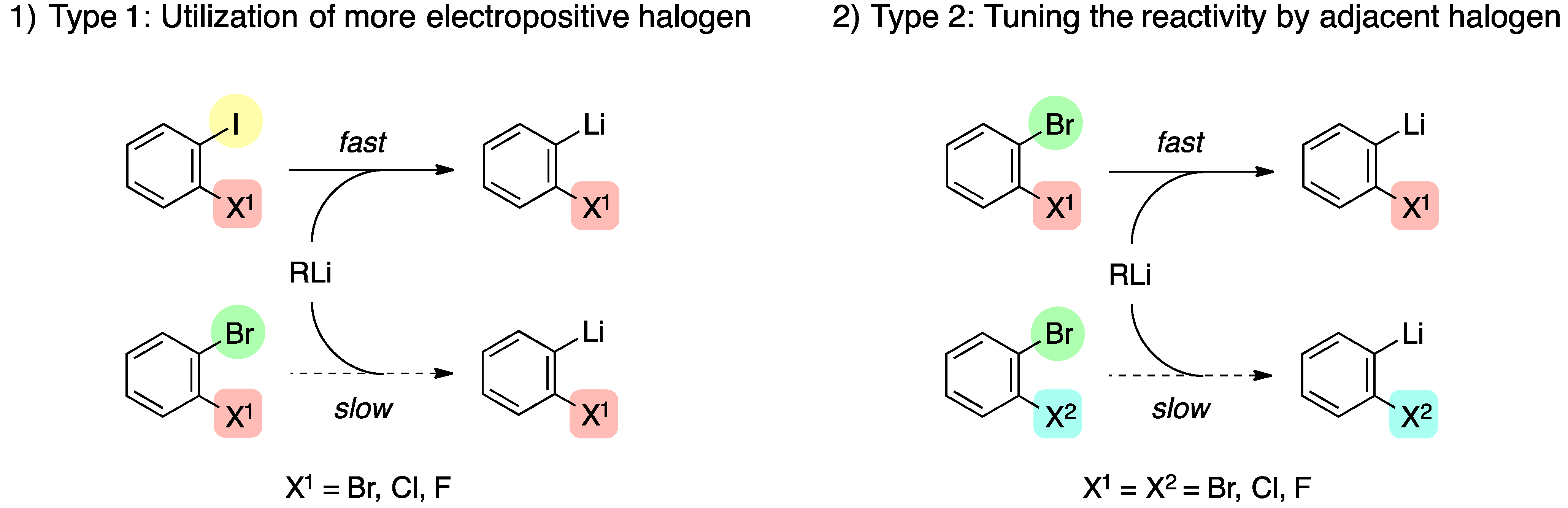 Source: mdpi.com
Source: mdpi.com
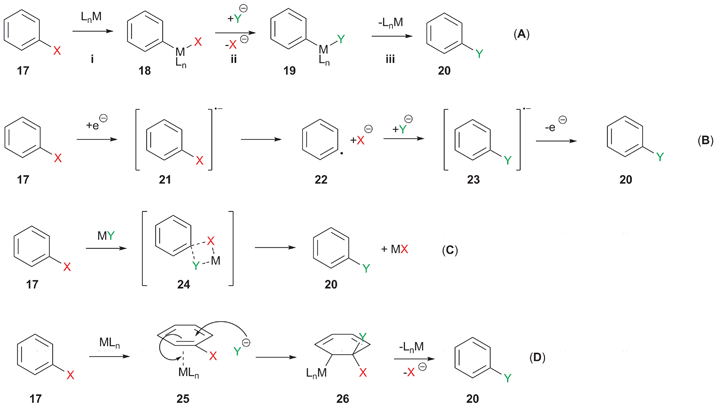
Mechanistic Postulates for Li-Halogen Exchange Electron transfer radical process R1XR2M Nucleophilic mechanism via halogen ateÐtype intermediate. Anomalous aromatic substitution via halogen-metal exchange 211 Since in the halogenmetal exchange reaction lithium is introduced in the aromatic ring at the position where the halogen was present the method is used to obtain the aryllithium compounds regiospecifically. In organometallic chemistry metalhalogen exchange is a fundamental reaction that converts a organic halide into an organometallic product. Aryl and Heteroaryllithium Compounds by Metal - Halogen Exchange. An obvious solution is a transition-metal-catalysed process which could operate via a variety of mechanisms including both one- and two-electron pathways Scheme 3 Paths AD.
 Source: europepmc.org
Source: europepmc.org
When the metalhalogen exchange temperature was set to 78 C. Remarkably efficient processes have been developed for metal-mediated halogen exchange in aryl and vinyl halides. In the above reaction the Grignard reagent cannot simply replace phenyllithiun some Mg cases have been reported for other halides but Li is the stalwart metal. Synthesis of Carbocyclic and Heterocyclic Systems. A viable process for aromatic halogen exchange must therefore proceed via an alternative mechanism.
 Source: chem.ucla.edu
Source: chem.ucla.edu
Tert-butyl magnesium bromide a Grignard reagent can be prepared by halogen-metal exchange. If some halogen exchange reactions are possible with activated substrates they usually require catalysis with metal complexes. Knochel and coworkers have demonstrated the functional-group tolerance of magnesium-halogen exchange which is now the method of choice for the preparation of highly functionalized organomagnesium reagents. Prepared by metal-halogen exchange. An obvious solution is a transition-metal-catalysed process which could operate via a variety of mechanisms including both one- and two-electron pathways Scheme 3 Paths AD.
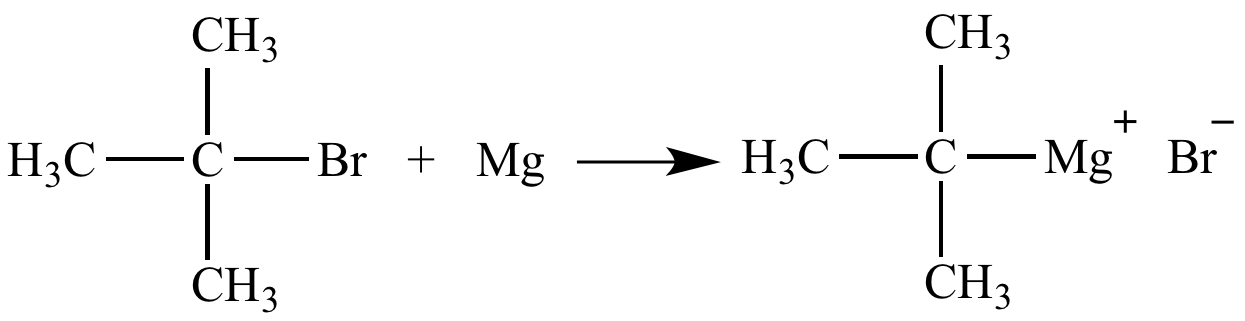 Source: chem.ucla.edu
Source: chem.ucla.edu
The yields of the newly formed Grignard and the final product are substantially improved in comparison to the identical reaction without a ligand additive. The possibility that haloarenes containing an ortho functional group may undergo metalhalogen exchange with Ph 2 CuLi LiCN to form ortho-substituted metallated aryl reagents and the potential of this process for subsequent reactions with electrophiles has been investigatedThe procedure occurs under very convenient conditions and is potentially suitable for arenes which may. In the presence of the tridentate ligand both the metalhalogen exchange and the reaction with the electrophile can be performed at 10 to 25 C. European Journal of Organic Chemistry 1998 1998 9 1851-1860. An obvious solution is a transition-metal-catalysed process which could operate via a variety of mechanisms including both one- and two-electron pathways Scheme 3 Paths AD.
 Source: pubs.rsc.org
Source: pubs.rsc.org
45-Bromoimidazole gave a mixture of 4- and 5-bromo-1-methylimidazole on treatment with 1 or 2 mol equiv. Today this reversible lithium-halogen exchange reaction can be formulated as R L i R X K R L i R X. When Mg is added to tert-butyl bromide the carbon-bromine bond is exchanged for a carbon-magnesium bond. The reaction commonly involves the use of electropositive metals Li Na Mg and organochlorides bromides and iodides. Remarkably efficient processes have been developed for metal-mediated halogen exchange in aryl and vinyl halides.
 Source: researchgate.net
Source: researchgate.net
Synthesis of Carbocyclic and Heterocyclic Systems. Today this reversible lithium-halogen exchange reaction can be formulated as R L i R X K R L i R X. In the above reaction the Grignard reagent cannot simply replace phenyllithiun some Mg cases have been reported for other halides but Li is the stalwart metal. X is a halogen atom Cl Br or I. In organometallic chemistry metalhalogen exchange is a fundamental reaction that converts a organic halide into an organometallic product.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
If some halogen exchange reactions are possible with activated substrates they usually require catalysis with metal complexes. Whereas without a transition metal a large excess of Et 2 Zn 50 equiv is required to perform an iodinezinc exchange 22 the addition of CuI 03 mol reduces the amount to 15 equivalents. The possibility that haloarenes containing an ortho functional group may undergo metalhalogen exchange with Ph 2 CuLi LiCN to form ortho-substituted metallated aryl reagents and the potential of this process for subsequent reactions with electrophiles has been investigatedThe procedure occurs under very convenient conditions and is potentially suitable for arenes which may. Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi. A viable process for aromatic halogen exchange must therefore proceed via an alternative mechanism.
 Source: orgmech.co.uk
Source: orgmech.co.uk
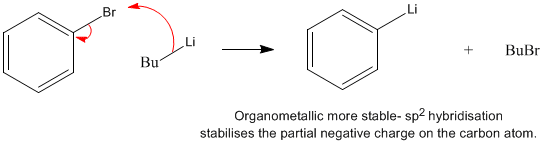
This stereospecificty is highlighted in the lithium-halogen exchange of the vinyl halide shown below. The possibility that haloarenes containing an ortho functional group may undergo metalhalogen exchange with Ph 2 CuLi LiCN to form ortho-substituted metallated aryl reagents and the potential of this process for subsequent reactions with electrophiles has been investigatedThe procedure occurs under very convenient conditions and is potentially suitable for arenes which may. Perhaps the most logical approach is a typical cross-coupling. Particularly well-developed is the use of metalhalogen exchange for the preparation of organolithium compounds. Lithium-halogen exchange is extremely fast.
 Source: slideplayer.com
Source: slideplayer.com
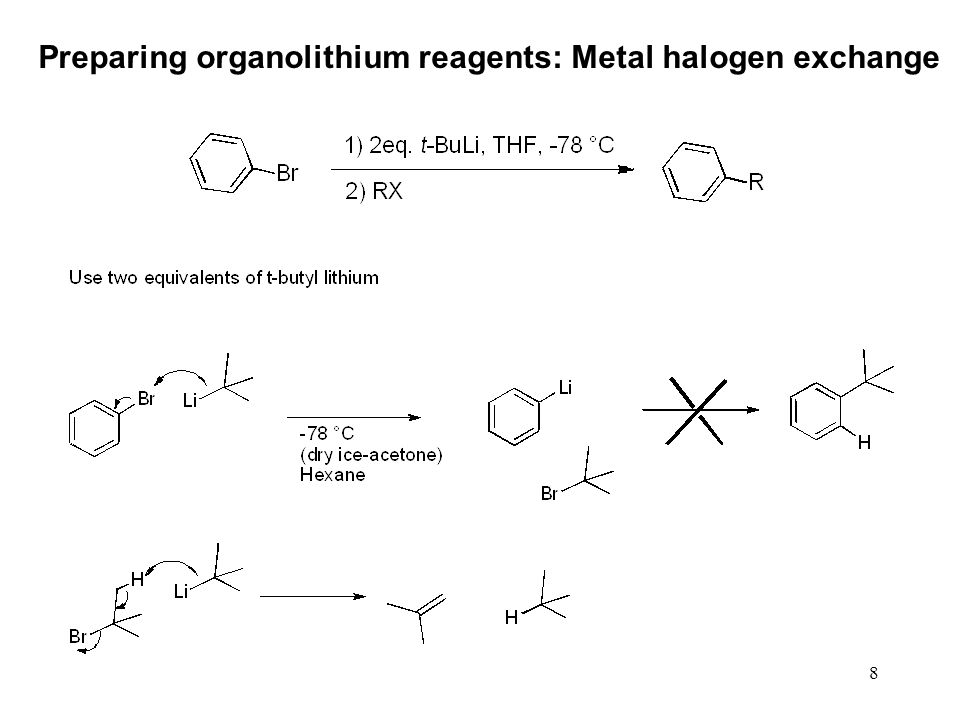
Brian Iddon and Bee Lan Lim Abstract. Magnesium-Halogen Exchange Chem 115 Jason Brubaker Review. A viable process for aromatic halogen exchange must therefore proceed via an alternative mechanism. The byproduct formed in the case of 2b implies that the. Lithium-halogen exchange is extremely fast.
 Source: mdpi.com
Source: mdpi.com
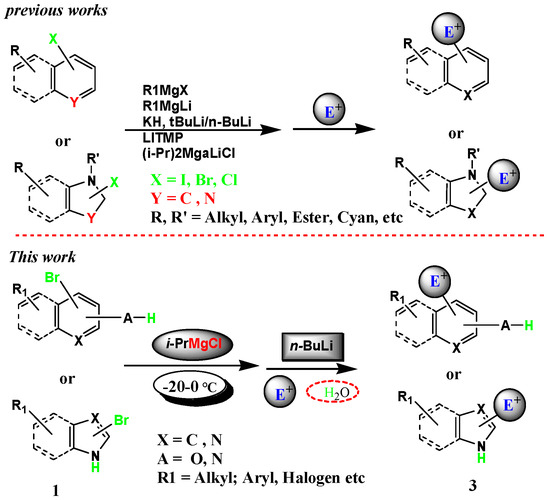
The Role of Ate Complexes in HalogenMetalloidMetal Exchange Reactions. Lithium-halogen exchange is extremely fast. As an equilibrium process we know that the more. This stereospecificty is highlighted in the lithium-halogen exchange of the vinyl halide shown below. Metalhalogen exchange reactions of mono- and poly-halogenoimidazoles.
 Source: finder-articles.com
Source: finder-articles.com
Metalation is a common way of preparing versatile organolithium reagents. Lithium-halogen exchange is extremely fast. A viable process for aromatic halogen exchange must therefore proceed via an alternative mechanism. 1 Common metalation reagents are the butyllithiums. The possibility that haloarenes containing an ortho functional group may undergo metalhalogen exchange with Ph 2 CuLi LiCN to form ortho-substituted metallated aryl reagents and the potential of this process for subsequent reactions with electrophiles has been investigatedThe procedure occurs under very convenient conditions and is potentially suitable for arenes which may.
 Source: researchgate.net
Source: researchgate.net
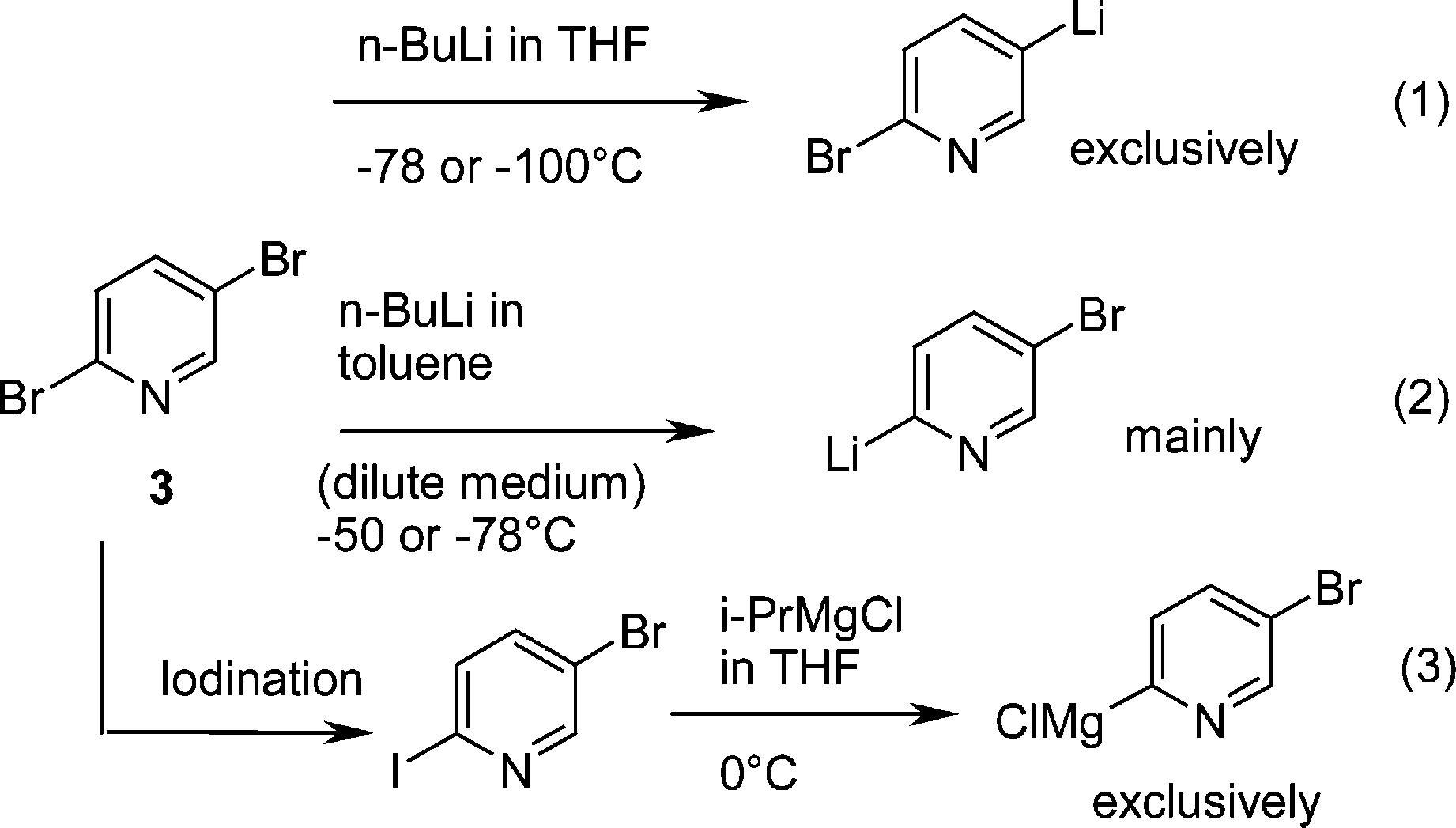
Remarkably efficient processes have been developed for metal-mediated halogen exchange in aryl and vinyl halides. If the reaction temperature of the metalhalogen exchange was set to 78 C first and then warmed up to 30 C for 3 h only 3b could be generated in 70 yield Table1 entry 3. Of n-butyl-lithium in ether or THF under various reaction conditions followed by addition of dimethyl sulphate. Intermediates in the metal-halogen exchange. Lete AffiliationDepartamento de Quimica Organica II Facultad de Ciencias Universidad del Pais Vasco Apartado644 48080 Bilbao Spain KeywordsHeteroaryllithium Compounds Halogen Exchange Carbocyclic sporadic.
 Source: pubs.rsc.org
Source: pubs.rsc.org
Intermediates in the metal-halogen exchange. They are overviewed in a comprehensive manner in this review article. As an equilibrium process we know that the more. Tert-butyl magnesium bromide a Grignard reagent can be prepared by halogen-metal exchange. In organometallic chemistry metalhalogen exchange is a fundamental reaction that converts a organic halide into an organometallic product.
 Source: frontiersin.org
Source: frontiersin.org
Tert-butyl magnesium bromide a Grignard reagent can be prepared by halogen-metal exchange. In some instances the rate of lithium-halogen exchange can exceed the rate of. Synthesis of Carbocyclic and Heterocyclic Systems. Of n-butyl-lithium in ether or THF under various reaction conditions followed by addition of dimethyl sulphate. 5-lodo- and 24.
Source: chemicalforums.com
The possibility that haloarenes containing an ortho functional group may undergo metalhalogen exchange with Ph 2 CuLi LiCN to form ortho-substituted metallated aryl reagents and the potential of this process for subsequent reactions with electrophiles has been investigatedThe procedure occurs under very convenient conditions and is potentially suitable for arenes which may. Of n-butyl-lithium in ether or THF under various reaction conditions followed by addition of dimethyl sulphate. Today this reversible lithium-halogen exchange reaction can be formulated as R L i R X K R L i R X. If the reaction temperature of the metalhalogen exchange was set to 78 C first and then warmed up to 30 C for 3 h only 3b could be generated in 70 yield Table1 entry 3. Mechanistic Postulates for Li-Halogen Exchange Electron transfer radical process R1XR2M Nucleophilic mechanism via halogen ateÐtype intermediate.
 Source: www2.chem.wisc.edu
Source: www2.chem.wisc.edu
Metalation is a common way of preparing versatile organolithium reagents. Gilman suggests a nucleophilic mechanism. If some halogen exchange reactions are possible with activated substrates they usually require catalysis with metal complexes. Particularly well-developed is the use of metalhalogen exchange for the preparation of organolithium compounds. Anomalous aromatic substitution via halogen-metal exchange 211 Since in the halogenmetal exchange reaction lithium is introduced in the aromatic ring at the position where the halogen was present the method is used to obtain the aryllithium compounds regiospecifically.
 Source: youtube.com
Source: youtube.com
Prepared by metal-halogen exchange. They are overviewed in a comprehensive manner in this review article. Transition-Metal-Catalyzed HalogenZinc Exchange Reactions. A viable process for aromatic halogen exchange must therefore proceed via an alternative mechanism. Prepared by metal-halogen exchange.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title metal halogen exchange by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.